Cormedix Inc Completes Review and Source Verification of Phase 3 Lock-it 100 Data for Neutrolin(R)
In this article I will nowadays some basic facts almost catheters, how CRMD seeks to address catheter related bloodstream infections with Neutrolin, and refute many claims about CRMD and Neutrolin made in a recent article past The Pump Stopper.
What is a Catheter
Many times during the course of care of a patient, physicians and nurses volition require reliable access to a patient's bloodstream. This admission may be necessary to deliver treatment in the course of medications and fluids, perform dialysis, monitor the patient with specialized instrumentation, and even evangelize diet in the form of Total Parenteral Nutrition (TPN). Catheters are used in both the inpatient setting, such every bit in the ICU, too equally in the outpatient settings such equally in dialysis and oncology (cancer) clinics or simply for the continuation of care initiated in a hospital (delivery of antibiotics for example). While these catheters perform a very critical function, unfortunately since they are placed directly into the bloodstream they are too source of hospital acquired and outpatient infections.
The Problem: Catheter Related Bloodstream Infections
Catheter infections occur in direct relation to the duration of catheter implantation. Figures regarding infection rates are usually reported as the number of infections per 1000 days of catheter use. These take been reported to range from 2-v infections/1000 days. These infections create significant problems and costs for the patient and medical arrangement. Several hundred k patients develop infections equally a result of indwelling catheters each year in the U.South. (see here). Direct infirmary costs are estimated to exceed over iii billion dollars. Furthermore in sure patient populations, up to 25% mortality has been reported.
CorMedix'southward (NASDAQ:CRMD) solution: Neutrolin
Information technology is against this back-drop that CRMD is poised to bring the first FDA approved catheter lock solution to market. A catheter lock solution is whatsoever solution placed into the catheter that is designed to go along the lumen of the catheter sterile and open during the periods that the catheter is NOT in use, otherwise known as its "locked" state. Hence the proper noun "catheter-lock-solution." Current standard of care despite years of exploring other alternatives remains the use of a simple heparin lock solution. Heparin has limited, if any, antimicrobial properties and predominantly serves to protect the lumen of the catheter from occlusion by a claret clot.
CRMD'south production, Neutrolin, consists of a solution of Taurolidine-Heparin-Citrate. This proprietary and patented solution has a unique blend of anti-thrombotic and anti-infective properties. A like product has been manufactured in Europe by TauroPharm, a contract pharma manufacturer turned competitor that CRMD is litigating for contractual violations and patent infringement. A final court conclusion is still pending in these cases. Later on its own CE mark was obtained, this TauroPharm "knockoff" has enabled over a dozen randomized and descriptive studies to be performed. These have uniformly shown highly significant reduction of catheter infection rates of 60-95%, still without the evolution of drug resistance or any safety bug. It is based on these clinical and other safe studies that the FDA canonical a stage 3 report of Neutrolin slated to begin in the fourth quarter of 2015. CRMD has engaged Evercore to explore strategic alternatives including possible partnership or auction in gild to expedite bringing Neutrolin to market place. This procedure is non yet consummate.
The Long Case for CRMD
CRMD presents a compelling long position based on the hope of Neutrolin. Equally I have published hither, I believe odds of a stage 3 success for Neutrolin are exceedingly good. I invite you to read that article for a more complete clarification of the upcoming phase three report and why I believe it will be successful. If successful, it is virtually certain that Neutrolin will replace heparin as the standard of intendance locking solution for most indwelling catheters in the U.Southward. Each yr in the U.S. alone, betwixt five and 7 million catheters are placed in patients indicating a huge addressable market.
Given the all-encompassing apply of catheters in medicine and the multiple treatments required during a catheter'southward lifetime, even at overly conservative pricing ($x-15/treatment) potential revenue from Neutrolin would be significant. Based on a conservative 25-40 one thousand thousand catheter days/year in the U.South., revenue from Neutrolin runs into the multiple hundreds of millions of dollars per twelvemonth, if non even into the billions under less conservative market scenarios. Given a fully diluted market cap of simply roughly $175 1000000 at this time and the possibility of a product that could bring in that kind of revenue for 10.five years (current length of market exclusivity period granted by the FDA), or even longer if certain orphan drug status designations are achieved, I see this as a very expert run a risk/reward state of affairs. Other expert sources of information on CRMD and its history, potential, and progress can exist found in this article past Wall Street Teacher and this commodity by Mark M.
Brusque Article on CRMD, Neutrolin
On June 29, 2015, an author who goes by the title The Pump Stopper published an article on Seeking Alpha titled: "CorMedix: Strong Sell on Partner Failure, Misleading Data And Paid Stock Promotion, -91% Downside." In this article the author made a number of unsubstantiated claims most CRMD and its lead product Neutrolin. While the author made a significant number of assertions, this is not the forum to dispute every concluding item of his article. Instead, I will focus on a few important claims. The reader can then decide based on this presentation of the facts if The Pump Stopper deserves any trust for any claim he presented against CRMD. The claims I will address today include:
- Centers for Disease Control (CDC) and Infectious Disease Society of America (IDSA) recommend other catheter locking solutions as standard of care making Neutrolin unnecessary.
- CRMD has fabricated misleading claims about Neutrolin, particularly since other more than clinically constructive solutions for the prevention of catheter related claret stream infection already exist in the U.S.
- Heparin is not the current standard of care locking solution used in the U.South. and therefore is an inappropriate comparator for the purposes of CRMD's upcoming pivotal Stage 3 studies. Using heparin unnecessarily places patients at risk.
- I of the key components of Neutrolin (taurolidine) has a toxic breakup product, formaldehyde. CRMD has been negligent in failing to fairly report the effects of this breakdown product.
- Neutrolin is not even vaguely toll constructive against current established medical protocols.
- CRMD's partnership process managed past Evercore has failed.
- CRMD is at present "targeting" retail investors with paid stock promotion.
- CRMD's management failed to make advisable disclosures regarding recent suits brought past CRMD confronting TauroPharm in Germany.
For this department of the commodity I volition adopt the following format: Beginning I will elucidate the author'southward claim. Then I volition nowadays the truth. Finally, I will reveal the writer's method. Through this process I believe the reader will exist able to clearly see that the information The Pump Stopper presents as ostensibly well researched and scientifically sound are well-nigh all inappropriately quoted or taken out of context. I will let the reader postulate motives for these activities.
Merits: Both the Centers for Illness Control and Communicable diseases Society of America recommend other catheter locking solutions as standard of intendance making Neutrolin unnecessary.
Truth: Neither the CDC nor the IDSA have made any recommendation for any agent every bit a routine catheter locking solution beyond the current standard of care heparin. CRMD, via Neutrolin, seeks to fill up a significant healthcare void in the U.Due south. The truth is that there are approximately 500,000 cases of hospital acquired bloodstream infections each year in the U.South., and a vast majority of serious blood stream infections are catheter related infections (see here). These infections cause meaning patient morbidity and mortality, and they are a significant cost to the healthcare system besides. Estimates of treatment price per infection case are $33,000 to $75,000 with hospitals suffering a loss due to unreimbursed care in excess of $26,000/case. Despite the writer's claims, in the U.S. in that location is a very real and significant need for a catheter locking solution to replace heparin every bit standard of care.
Method and exhibits:
The author states: "Both antibiotic and many non-antibody compounds proven to be effective and cheap. These are all recommended by the CDC…." However, the author fails to provide a single reference from the CDC to a single recommended solution. Since he fails to provide any links to support his claim, I will provide the CDC link here. The recommendations for care of central venous catheters start on page eleven. I'll offer you read the recommendations to come across if the author'southward claims are correct. Yous will actually find that there is in fact no recommendation for routine utilise of antibiotic locking solutions. The only time the CDC recommends antibody locking solutions is "in patients with long term catheters who have a history of multiple CRBSI despite optimal maximal adherence to aseptic technique [120- 138]. Category 2"
Next The Pump Stopper offers the reader the following quotes from this text: 
If you become look at the actual text however, he has completely failed to accurately represent the statements. Subsequently the get-go quote above regarding clinical practice guidelines, the rest of the paragraph in that same paper actually states: " Guidelines from the Centers for Illness Control and Prevention recommend ALT as prophylaxis for patients with long-term catheters and a history of multiple CRI despite maximal efforts to follow aseptic technique ." Can you encounter what the author simply did? He left off the qualifying half of the CDC recommendation. This half of the argument is critical every bit information technology shows that the only time antibiotic locking solutions are recommended past the CDC is in very limited and focused circumstances. Utilise is not recommended for prophylactic employ.
Here is the actual section of the text The Pump Stopper purported to accurately quote in its entirety so you can run into for yourself:

You can see above that The Pump Stopper so again cherry picks merely half of a quote regarding the Guidelines from the IDSA. Compare what he presents (2nd quote above) vs how it actually reads:
"Guidelines from the Infectious Diseases Society of America for the diagnosis and direction of CRI recommend antibiotic locks as adjunctive therapy specifically for catheter salvage in cases where the catheter is non removed." The writer left off the 2d half of the judgement which completely negates his bespeak (no, your eyes are not deceiving y'all). The actual IDSA recommendation is for catheter salvage and only when catheters are non able to be removed. Reverse to what the author claims, IDSA did not issue a recommendation for routine use of antibiotic locking solutions.
Quote iii is exactly the reason we demand Neutrolin. When patients must take an infected catheter removed, it increases the cost of their care, delays necessary treatments, and increases morbidity and mortality. If we have a solution available for use in all catheters as a means of preventing the infection in the first place, nosotros wouldn't take to remove so many catheters. 
Claim: CRMD has made misleading claims about Neutrolin, especially since other more than clinically effective solutions for the prevention of catheter-related blood stream infection already exist in the U.S.
Truth: CRMD has not made any misleading claims about Neutrolin. There are in fact no FDA approved or even any widely used "off characterization" catheter lock solutions. The standard of care is heparin. In some circumstances antibiotics are used inside the lumen of a catheter as a "lock," however, this is typically to treat an already present catheter related infection. Neutrolin is not being proposed for handling of infection, it is being advance by CRMD for prevention of infections. At that place are several important reasons antibiotic based locking solutions are not widely used. These include: (1). Development of antibody resistance when used in a prophylactic mode; (2). Systemic toxicity; (iii). Frequent lack of effectiveness in one case a biofilm has formed. Other locking solutions such equally ethanol may have weaker antibacterial effect or gamble causing catheter deposition. The fact is at that place is no other solution that effectively addresses the unmet demand Neutrolin proposes to treat.
Method and exhibits:
The author makes a number of claims confronting CRMD that nosotros need to accost here. To make his case that other (improve) solutions already exist in the U.S. he states: " The reason for this is that a simple and virtually free Gentamicin flush reduces infections by 92%, which is really more some of CRMD's efficacy claims." He and then proceeds to mail service the following slide to back up his merits:
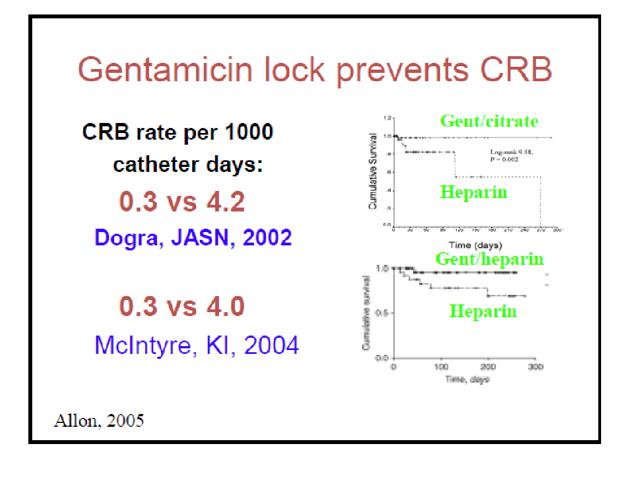
However, he and so omits to include the following two slides constitute here in the more complete version of this presentation (which he links later and obviously knew almost when preparing this article):
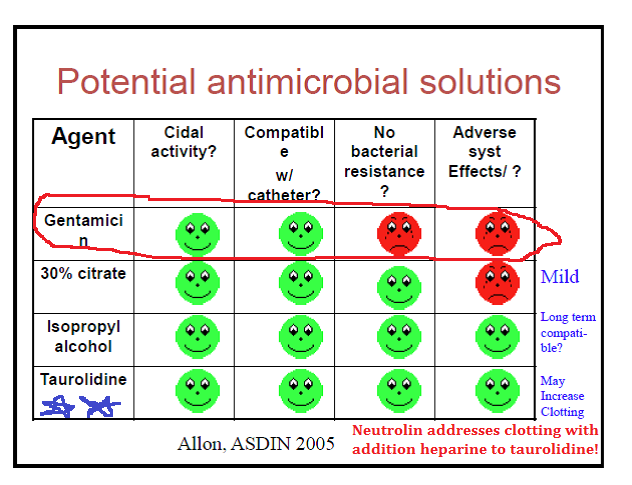
and…
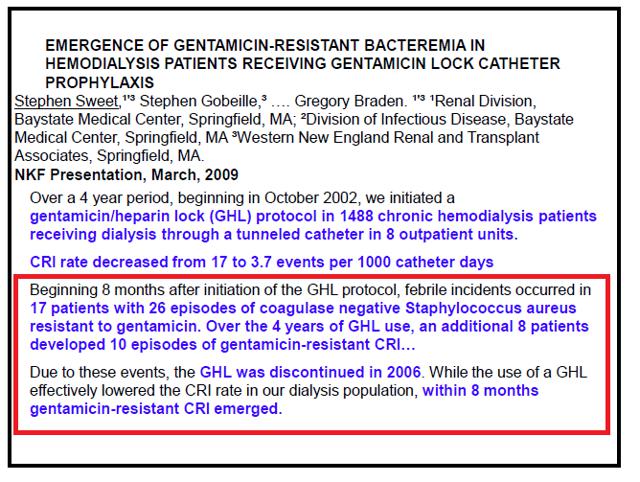
Why is this of import? If y'all read the text in the slide, information technology states:
"Due to these events, GHL (Gentamicin heparin lock) was DISCONTINUED in 2006. While use of a GHL finer lowered the CRI (catheter related infection) charge per unit in our dialysis population, within 8 months gentamicin-resistant CRI emerged."
Surely, with this information, nosotros accept to recognize the medical community is non routinely using gentamicin locking solutions. In that location is conspicuously major room for improvement over antibiotics that have inherent risks of resistance development or other toxicities.
Next, The Pump Stopper states "Clinical Studies Evidence CRMD Production Offers No Benefit vs Current Industry Standard Protocols."
Actually, how can this exist the case? We physicians oasis't even divers an "Industry Standard" yet across heparin…retrieve the CDC slides, remember we just showed that gentamicin lock was not the standard of care, etc. Anyhow, despite quoting from a presentation that actually showed gentamicin lock was "abandoned" by a group that had used information technology extensively over a flow of 4 years, the author continues to promote gentamicin locking solutions as current industry standard for the sake of his statement. He presents the following:

While the study quoted showed gentamicin/heparin to be similarly constructive to taurolidine/citrate over a short period of time in a pocket-sized group of patients, the comparison The Pump Stopper draws to Neutrolin is just as flawed as the writer's championship for this department quoted in a higher place is. The study in a higher place did not compare gentamicin to Neutrolin. Neutrolin is not a combination of taurolidine/citrate, information technology is actually a combination of taurolidine, citrate and heparin. Heparin is a critical additional ingredient equally it provides substantial benefits including less risk of catheter thrombosis, and it may also help in prevention of biofilm. Data for these assertions are in part based on CRMD's own ongoing Neutrolin Apply Monitoring Program (NUMP) as presented at multiple renal conferences where it appears to provide fifty-fifty greater reduction of infection. This NUMP study is demonstrating that infection reduction is actually 96% vs heparin during this ongoing and continuously updated program. For independent verification observe the actions of TauroPharm (a visitor in Germany with no U.Due south. presence) which really in November 2007 started adding heparin to their similar taurolidine and citrate based solutions.
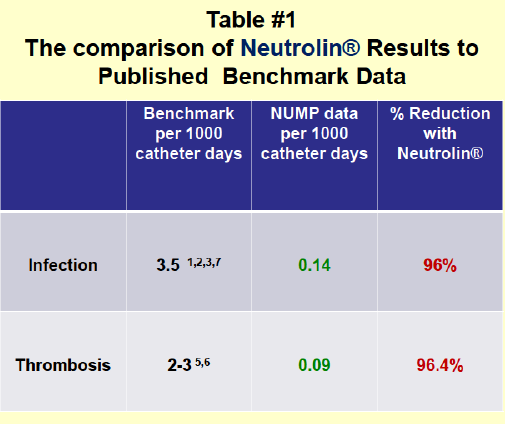
Source: Information from the Neutrolin Use Monitoring Program
Additionally, as we've pointed out, antibiotic locks are subject to creation of problems regarding the development of antibody resistance. Neutrolin contains taurolidine as i of its active ingredients which is unique because it is non an antibody…instead it has inherent antimicrobial properties that address multiple types of infections including bacteria and fungus. Thus taurolidine has never been show to showroom human microbial resistance (see Wikipedia page here for more than data on taurolidine and how it is unique). For the author to state Neutrolin would provide "no benefit" over current protocols is clearly incorrect. Simply the fact that microbial resistance is not an issue with Neutrolin is a HUGE advantage, and the reason I believe Neutrolin will become the new standard of care in the U.s.a. once canonical.
The Pump Stopper states: Current Catheter Lock Protocols Penetrate Biofilms "Rapidly" and "Completely." Problem for The Pump Stopper's statement hither consists in the fact that CRMD is non claiming Neutrolin penetrates a biofilm. However one can learn a few things from how the author presents his version of the facts. The case for Neutrolin has never been that it "penetrates" a biofilm. The claim is that it helps to "foreclose" biofilm formation. Come across these photographs below comparing catheters with heparin (extensive biofilm) on the left vs Neutrolin (no biofilm) on the right.
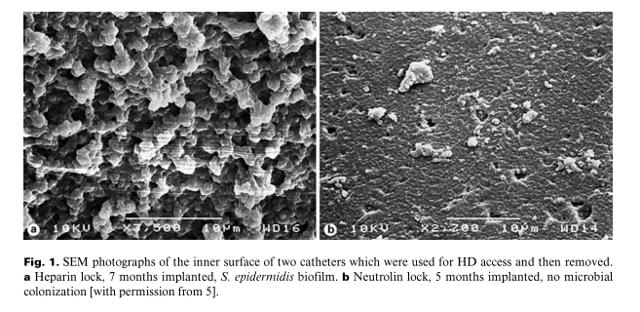
By the fourth dimension a substantial biofilm has formed and the patient gets an infection, the catheter typically has to be removed. Further in the article the author erroneously cites, the text actually states: "The multi-factorial nature of biofilm resistance to antibiotics is well-illustrated by data suggesting biofilm penetration is unrelated to the ability of the antibody to disrupt biofilm or kill biofilm cells." And so even though he has presented an unsubstantiated argument confronting Neutrolin (penetration of the biofilm), he compounds his error by failing to disembalm that biofilm penetration by antibiotics does not necessarily issue in effective biofilm disruption. Furthering this misguided idea procedure, the author states: "there is a multitude of other non-antibiotic protocols already in use for years which finer deal with biofilms." Actually? If that is the case, why do we carp removing infected catheters at all?
Merits: Heparin is not the current standard of care locking solution used in the U.Southward. and therefore is an inappropriate comparator for the purposes of CRMD'due south upcoming pivotal Phase 3 studies.
Truth: Heparin is in fact the electric current standard of care catheter locking solution in the U.South. and is the virtually advisable comparator for CRMD'due south Phase 3 studies. The FDA approved the Phase iii study comparing Neutrolin to heparin with a guideline that Neutrolin needed to show a xl% reduction in infection vs heparin. Additionally, there is in fact no FDA approved or even widely used "off label" catheter lock solution in the US today. Despite the authors claims antibiotics are used as a "catheter lock" in patients who already have an infection, or in certain loftier take a chance patient (example those who've had prior catheter related infections). There are multiple reasons there is non wide spread utilise of antibiotics in catheter locking solutions including risk of systemic toxicity, lack of effectiveness one time a biofilm has formed and virtually importantly due to the finding that antibiotic solutions contribute to the development of antibiotic resistance when used in a preventative fashion.
Neutrolin's ingredient taurolidine is non an antibiotic, it is an antimicrobial substance. For this reason it has multiple advantages. 1. Resistance in homo has never been demonstrated. ii. It has broad spectrum activity preventing both bacterial and fungal infections. 3. Even at excessively high doses (well more than than one could exist exposed to in the form of normal treatment) it has not been demonstrated to take whatever serious systemic side effects.
Method and exhibits:
First the author states: "a elementary heparin catheter lock is NOT the standard for infection treatment and CRMD'due south comparison and proposed Phase three trial confronting this irrelevant benchmark seems very misleading to me."
The facts are that a heparin catheter lock is Non the standard for handling infection. The author is correct, no one would propose to utilise heparin to "treat" whatever infection. Furthermore, CRMD is not proposing Neutrolin as an infection treatment either. Every bit nosotros reviewed above, treatment of infections per the CDC normally involves removal of the catheter except when this is not possible. Neutrolin is designed for PREVENTION; before an infection e'er takes place.
The author continues to belabor the bespeak saying: "If CRMD'due south Stage iii trial was properly constructed and CRMD forced to compare its worthless Taurolidine solution against what doctors actually use for catheter infections."
Wait…when did CRMD ever propose to "care for" infections with Neutrolin? Never.
The author continues with this approach even so, even accusing CRMD of willfully putting patients at hazard of impairment. The reader might exist asking themselves at this bespeak "Would the FDA approve CRMD'southward phase three written report if there was already an established protocol for this indication?" The answer is no, only the author apparently wants you to believe they have uncovered something the FDA failed to consider. The Pump Stopper writes:

The author'south reference to cancer trials farther shows his lack of understanding of how clinical trials are constructed. In all fields of medicine, when at that place is no defined standard of care, a placebo is frequently exactly what y'all compare to. Don't believe me, look at this paper that addresses the very consequence of placebo's in oncology studies. Of course when constructive treatment exists that will be offered, but if there is no clear standard of care, a placebo tin and is used, fifty-fifty in sure cancer studies. In CRMD'south phase 3 study, the control arm is in fact the electric current standard of intendance: heparin. Heparin would never exist considered a placebo (or sham handling like a saccharide pill).
Claim: One of the key components of Neutrolin (taurolidine) has a toxic breakdown production, formaldehyde. CRMD has been negligent in failing to adequately study the effects of this breakup product.
Truth: 1 of Neutrolin'southward key ingredients is taurolidine. Taurolidine does in fact take a breakdown product formaldehyde. CRMD has non been shy about this, even putting this in their ain presentations to members of the medical community, for example in this presentation to the attendees of the National Kidney Foundation conference held in Dallas, Texas in late March 2015. CRMD is hiding nix even from a briefing of physicians because the truth is it has nothing to hide. Your ain body is constantly making and metabolizing formaldehyde in low levels (see the American Cancer Club'due south website discussion of formaldehyde here). The concentrations of formaldehyde in the lumen of the catheter that actually become into the claret stream are thus probable inconsequential. Furthermore, taurolidine has even been investigated as a potential anti-neoplastic agent (anti-cancer therapy) as discussed here. Taurolidine is being investigated for a number of potential indications as an anti-neoplastic agent for case here, hither, and here. CRMD has no need to perform carcinogen studies on a chemical compound that for decades has been documented to be safe even at doses substantially college than in Neutrolin.
Method and exhibits:
The Pump Stopper implies that CRMD has somehow been negligent in its Phase 3 written report blueprint, fifty-fifty suggesting CRMD should exist doing carcinogen studies. As you lot can meet from but the few examples linked above, there are plenty of studies being done on the possible anti-neoplastic effects of taurolidine. Again, the facts prove the exact reverse of what the author seeks to imply.
Claim: Neutrolin is not even vaguely cost effective against current established medical protocols.
Truth: Beginning, as we have mentioned there is no established effective protocol in the U.South. that addresses the prevention of catheter related bloodstream infections. Any such comparisons would therefore be impossible. Second, the author fails to recognize the substantial costs that are associated with catheter related bloodstream infections. Hospitals on average lose tens of thousands of dollars for direction of each catheter related infection. Costs include the need for invasive surgical procedures to replace infected catheters, longer infirmary stays with more complicated treatment courses, potential demand for expensive antibiotics if resistant bacteria are isolated, etc. This doesn't fifty-fifty begin to accept into consideration the incalculable loss associated with increased patient morbidity and mortality.
Method and Exhibits:
Permit's look adjacent at the economics of Neutrolin as the author sees them. He includes the following prototype:

The author claims that gentamicin locking solution would toll no more than than $half dozen.77/dose. He then goes on to add in this image from the Middle East Dialysis presentation:

Well, two problems with the author's approach. First, gentamicin is an inappropriate comparison. It is not standard of intendance (was actually abased for this purpose) as we take proven and therefore has no begetting on what the cost of Neutrolin will be. 2nd, to cutting and paste a cost assay from a presentation in the Middle Due east and therefore assume the same market forces are at play in the U.S. is beyond naïve. I does not even pass the mutual sense exam. Does the author know what a drug similar Sovaldi retails for in the U.S.? It costs $84,000/treatment course before insurance discounts of probably about forty%. Now what does the exact same treatment protocol cost in a state like Egypt: $900 (see story here). In that location is over a fifty fold difference in price betwixt these regions. Certainly, in the U.S. the economics for Neutrolin will be much meliorate and Roth'due south estimates of $20+/lock application are not absurd as the author would endeavor to have yous believe. In fact the author's claim is and so off base in this regard, it fifty-fifty prompted the former Chairman of CRMD's board who resigned about 1 year ago to chime in on the comments department with this quote:

Claim: CRMD's partnership process managed by Evercore has failed.
Truth: CRMD's process with Evercore is even so underway. CRMD hired Evercore in club to "explore strategic alternatives to accelerate the evolution of Neutrolin" as discussed on multiple briefing calls. In the briefing call in March the CEO Randy Milby was asked about the timeline of the strategic process. Randy indicated the procedure could accept anywhere from 1-vi months.
The fact is, the process was projected to take up to 6 months, and that was in the initial stages of the process before Evercore had even had a chance to digest all the necessary information.
Method and Exhibits:
The author provides no evidence that the current process that Evercore is engaged in has failed. His sole footing for this claim appears to be that CRMD has non partnered to study Neutrolin in the past.
From author's article: 
Transcript of March conference call referencing the Evercore process:

Equally you tin see, CRMD never expected the process to be completed past June 29th, the engagement of The Pump Stopper'southward article and for the writer to merits the partnership process has failed at this bespeak is blatantly incorrect.
Claim: CRMD is now "targeting" retail investors with paid stock promotion.
Truth: CRMD has is not targeting retail investors with paid stock promotion. CRMD's share price rose from beneath $2 to only over $10 in a period of about 3 months with about no public activity past CRMD other than announcements regarding the FDA granting Neutrolin fast runway condition, FDA granting Neutrolin QIDP condition, and the CRMD 2014 briefing call held in March during with time CRMD appear hiring Evercore to explore strategic alternatives. The share price rose not because of any pumping activity by CRMD, just considering the value of Neutrolin rose significantly with these FDA designations. QIDP solitary (which the writer never once mentions in his article) grants Neutrolin an additional 5 years of U.Due south. market exclusivity. Additionally, Evercore is a world grade banker known for its merger and conquering activities.
Method and Exhibits:
The writer presents two texts he claims prove that CRMD is a pump operation. He depicts this image:

With this link, nosotros see that this "promotional" activity occurred in 2010. Most 5 years agone!!! More than significantly, the written report is very through and very informational and lacks any "pumping" characteristics. Proof of this is the share price for CRMD, which exhibited no significant change at the time of the report'south release. I claiming the reader to fifty-fifty find a cost target within the entire text. I looked hard and used a word search and couldn't even detect one. This isn't fifty-fifty close to a penny stock type promotion, pump-dump scheme despite what the author would like you to believe. Here is the start page clearly proving this report was released in Oct 2010 by the way:

Furthermore, to attempt to imply that this article from 5 years ago has had whatsoever effect on share toll today is ridiculous.
Additionally, if you look at the author's utilise of the Griffin Securities article, it is too from 2010, the year of the company's IPO, and CRMD was utilizing Griffin Securities for not-investment banking securities related services. Does that hateful this thorough article was evidence of a pump and dump scheme. Far from it. Many firms that employ banks and other entities for IPO's, secondaries or other purposes, subsequently receive coverage initiation from them.
I'd ask yous to go to CRMD's website and look at their press releases in the months leading up to and during CRMD's significant price appreciation. The only PR's you lot will find in the first few months of the year are those I accept just discussed. Nothing promotional in nature what so ever. For the author to merits the opposite is merely a deplorable reflection on the author.
Claim: CRMD'southward patent is a lost cause and management failed to make appropriate disclosures regarding recent suits brought past CRMD against TauroPharm in Germany.
Truth: CRMD is litigating against TauroPharm in Germany for violations of its proprietary information as well as violations of its utility model and patents that were granted for the novel employ of a taurolidine solution that besides contains citrate and heparin. These patents have been granted in both the U.Southward. and the EU. Most recently, CRMD sought an injunction against TauroPharm. A hearing was held on May eight, 2015 in the District Court of Mannheim. The courtroom declined to grant and injunction at that time and referred the determination back to the entities that granted the patents and utility model in the first place. CRMD disclosed to shareholders the finding of the District Court of Mannheim on the conference telephone call on the solar day of the hearing. Additionally, CRMD on May 19, 2015 released this 8-K further clarifying the District Court of Mannheim'southward determination after CRMD received written copies of the decisions.
Method and Exhibits:
The Pump Stopper claims that CRMD has failed to make appropriate disclosures regarding ongoing litigation in Germany regarding its patents and utility model. His exact statement reads:

The writer neglects to mention that in addition to the viii-K released on May 8 and the conference call on May viii (which they posted on their website here for anyone to hear and is besides bachelor in text format here), CRMD then on May 19, 2015 submitted an 8-K explaining in further detail the decision from the German courtroom in one case they received the written determination. There is simply no basis to the author's claim that CRMD has been negligent.
Conclusion:
CRMD has a potentially lifesaving handling in Neutrolin that fills a great unmet medical demand: prevention of catheter associated bloodstream infections. Neutrolin has been approved for a Phase 3 study and has been granted Fast Rails condition and QIDP designation. In that location is already substantial data available virtually its effectiveness via the European NUMP study, which therefore provides valuable data regarding the likelihood of success of the upcoming Phase 3 study. Multiple studies consistently demonstrate that the safe profile and efficacy of the Neutrolin locking solution is unmatched by any other locking solution available. Neutrolin has the potential to be quickly adopted equally the standard of intendance for catheter locking solutions upon FDA approval. With the vast number and types of catheters in employ in the U.S. today, Neutrolin is poised to generate substantial acquirement for CRMD or whatsoever future acquirer.
As you tin see, in this article I take presented a much different statement for CRMD and Neutrolin than The Pump Stopper presented. I have even used most of the exact same references, slides, and other texts that The Pump Stopper used in their commodity. It is truly impressive how different our conclusions are. I can only wonder why certain aspects of these texts and slides that back up CRMD's claims as well equally the need for and use of Neutrolin were omitted when The Pump Stopper wrote their article. I leave information technology to the reader to postulate why an writer admittedly short CRMD would go to such lengths to nowadays the textile to the public in such a way.
I volition offer i last consideration: For those contemplating an investment in CRMD, I can advise a very elementary method by which to determine which version of the facts to trust (mine vs The Pump Stopper's). Attempt this: phone call your local dialysis or oncology dispensary and ask whatsoever of the dialysis/infusion nurses the following question. "What catheter lock solution do you place in the catheter subsequently a treatment session for your routine catheter patients?" Notice out for yourself what the standard of care currently is, and thus yous will know if Neutrolin has the potential to be every bit big of a success as I expect information technology will.
Happy Investing.
This commodity was written by
I am a medical professional person that enjoys investing in stocks. My medical background I believe has prepared me well for the apply of technical analysis equally much of the skill of diagnostics in medicine is based on pattern recognition. I have a particular interest in biotechs with an ophthalmic (middle) focus as that is where I have developed the greatest expertise over my career and research. I hope over fourth dimension to share some of my professional insights with those of the Seeking Blastoff community in order to help them make more informed investing decisions. I await to learn a lot from others as well.
Disclosure: I am/nosotros are long CRMD. I wrote this commodity myself, and it expresses my own opinions. I am non receiving compensation for it (other than from Seeking Alpha). I take no concern relationship with whatsoever visitor whose stock is mentioned in this article.
Source: https://seekingalpha.com/article/3313875-cormedix-the-truth-about-neutrolin-future-standard-of-care
0 Response to "Cormedix Inc Completes Review and Source Verification of Phase 3 Lock-it 100 Data for Neutrolin(R)"
Post a Comment